EMEDEAS™
EMEDEASTM Early Medical Device Assessment
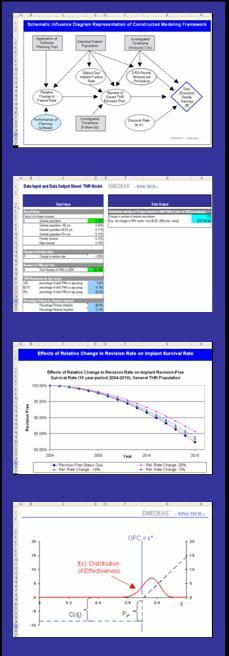
Based on state-of the art systems analysis methods and risk assessment tools, we incorporate clinical, technical, and market components into an integrated assessment and management tool. This allows us to develop jointly with our clients a comprehensive understanding of the most important drivers of patient benefit and product success.
Using our EMEDEAS™ framework, our clients are able to
- identifythe factors that are most critical to product success
- pinpoint and quantify uncertainties in various aspects of the technology and its application, and manage them accordingly
- perform scenario analyses and inform decision making at various stages of product development
Our clients have used our analyses to support the following decisions:
 Concept due diligence
Concept due diligence- Early-stage opportunity assessment
- Investment decisions
- Priority-setting for resource allocation
- Development and updating of product development plan
- Go- No-Go decisions
- Strategy development for targeted engineering, clinical, and market research
- Regulatory and reimbursement strategy
- Clinical trial formulation
- Product launch strategy
- Development of licensing terms
- Product pricing (health-economics/ cost-benefit)
- Portfolio management
Sample applications of the EMEDEAS framework have included:
- Musculoskeletal: New technology for hip joint replacement surgery
- Gastrointestinal: Evaluation and decision support for new optical screening technology
- Sleep Medicine: Strategy development for Obstructive Sleep Apnea (OSA) screening and therapy
